About synthesis and some transformations of carboranyl-containing derivatives of coumarine
Журнал: Научный журнал «Студенческий форум» выпуск №4(97)
Рубрика: Химия

Научный журнал «Студенческий форум» выпуск №4(97)
About synthesis and some transformations of carboranyl-containing derivatives of coumarine
Abstract. The investigated reaction with the metal derivatives of carboranes and secondary amines with 3-ethoxycarbonylmethyl. Based on the studied reactions, 4-isopropyl-o-carboranyl-, 4-morpholino- and 4-piperidino-3-ethoxycarbonyl-3,4-dihydro-coumarins were synthesized. The interaction of 3-ethoxycarbonyl-4-(isopropyl-o-carboranyl)-3,4-dihydrocumarin with amines was studied, on the basis of which various ammonium salts were obtained.
Keywords: coumarin, coumarin derivatives, 3-ethoxycarbonylmethyl.
Previously, it was shown [1-3] that 3-ethoxycarbonylcumarin, which has an activated double bond, is prone to nucleophilic addition reactions and, under the action of lithium- and magnesium-derived carborans, forms products of conjugated addition-3-ethoxycarbonyl-4-(R-o-carboranyl)-3,4-dihydrocumarins.
Developing studies of conjugate addition reactions in a number of coumarin derivatives, we studied the interaction of 3-ethoxycarbonylcoumarin with C-metal derivatives of carborans, natriimalon ether, nitromethane, and various amines.
Studies have shown that C-metal derivatives of isopropyl-o-carborane (Li, MgBr, Cu) interact with 3-ethoxycarbonylcumarin regiospecifically to form a conjugated addition product-3-ethoxycarbonyl-4-(isopropyl-o-carboranyl)-3,4-dihydrocumarin 1:

In the reactions of 3-ethoxycarbonylcumarin with morpholine and piperidine, conjugate addition is also observed leading to the amino derivatives 2,3:
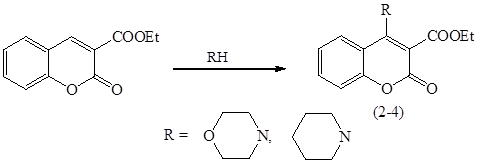
Interesting in theoretical and preparative relations are the reactions of 3-ethoxycarbonyl-4-(isopropyl-o-carboranyl)-3,4-dihydrocumarin 1 with methyl-, dimethyl- and trimethylamine, morpholine, piperidine and pyridine in an aqueous alcohol medium and ethanol, which, at a ratio of reagents 1:1, proceed regioselectively with the formation of ammonium salts 4-9:

The yields of ammonium salts in the case of morpholine are – 86, dimethylamine – 72, methylamine – 78, piperidine – 61 and pyridine – 56%, respectively, which indicates their connection with the basicity of amines and the possibility of side processes associated mainly with the destruction of the carborane core, observed in small quantities in the case of piperidine and dimethylamine, which are the strongest bases.
The structure of the synthesized compounds is confirmed by IR and NMR spectra, elemental analysis, and comparison with known samples.

