DETERMINATION OF ZIRCONIUM IN THE FORM OF MULTILIGAND COMPLEXES WITH ORTHONITROBENZOLAZOPYROCATECHIN AND CETYLTRIMETHYLAMMONIUM BROMIDE
Конференция: LXXXII Международная научно-практическая конференция «Научный форум: медицина, биология и химия»
Секция: Аналитическая химия

LXXXII Международная научно-практическая конференция «Научный форум: медицина, биология и химия»
DETERMINATION OF ZIRCONIUM IN THE FORM OF MULTILIGAND COMPLEXES WITH ORTHONITROBENZOLAZOPYROCATECHIN AND CETYLTRIMETHYLAMMONIUM BROMIDE
Abstract. The formation conditions of zirconium(IV) multiligand complexes with orthonitrobenzolazopyrocatechin and cetyltrimethylammonium bromide, as well as the mechanism of interaction between the reagent, metal, and surfactant, have been investigated. The complex formation reaction occurs in an acidic medium, and maximum absorbance is observed at pH = 1.5–4. The complex exhibits maximum light absorption at λmax = 480 nm. Upon introduction of the surfactant into the reaction system, orthonitrobenzolazopyrocatechin undergoes deprotonation, enhancing its ability to coordinate with the metal. The stoichiometric ratio of metal, reagent, and surfactant in the mixed-ligand complex was determined to be 1:2:2. The molar absorptivity of the complex is 2.8×10⁴ L·mol⁻¹·cm⁻¹, and the stability constant is βk = 7.1. The extractability of the complex using organic reagents for preconcentration and separation was studied. A single extraction with 500 μL of chloroform transfers 97.0% of zirconium into the organic phase as a mixed-ligand complex. High extraction efficiency is maintained within the temperature range of 10–40°C and aqueous phase volume of 20–40 mL, without a decrease in color intensity of the complex. Using the proposed method, microgram quantities of zirconium have been determined in natural and industrially significant materials.
Keywords: zirconium, extraction, orthonitrobenzolazopyrocatechin.
Introduction. Various physicochemical methods exist for the determination of zirconium [1, p.535, 2, p.736 3, p.3035]. However, many of the proposed methods have significant drawbacks. Selectivity is limited; the reagent may react not only with the target metal but also with interfering ions, and analytical procedures are often multi-step. The synthesis of complexes is time-consuming, instruments are expensive, and require special calibration. In the present study, the experiments conducted for zirconium determination are simple, the reagents are environmentally friendly, and no costly equipment is required.
Instruments and Reagents. The optical density of colored solutions was measured using a СФ-46 spectrophotometer or a КФК-2 photoelectrocolorimeter in a quartz glass cuvette with a thickness of l = 1.0 cm, and with a JENWAY 6300 spectrophotometer using a quartz microcuvette. For separation of the aqueous-organic phase, a Denley BS400 centrifuge (Denley Instruments Ltd., Billingshurst, UK) was used. The pH of the studied solutions was monitored using a Cond./TDS/Temp universal pH meter. Spectrophotometric measurements were carried out at 25.0±0.5°C.
Orthonitrobenzolazopyrocatechin was synthesized according to the procedure described in [4, p.383] and purified by recrystallization from alcohol. Using the titanometric titration method, the percentage content of each reagent was determined [5, p.386]. The purity of the reagent was 99.9%, and its molecular structure is as follows.

Method. A 5 µg amount of zirconium was added to a 10 mL test tube. Reagent and surfactant were then introduced, and the acidity was adjusted to the optimal pH using a buffer solution. Subsequently, extraction and dispersing solutions were added sequentially. Distilled water was added to bring the total volume to 10 mL. A turbid solution was obtained, which was shaken for 2 minutes and then centrifuged. The optical density of the concentrated and separated zirconium complex was measured using a spectrophotometer.
Results and Discussion. The pH of the sample solution significantly influences both the formation of the metal complex and its subsequent extraction into the organic phase. The effect of pH on complex formation and extraction was studied. Maximum absorbance of the complex at λmax = 480 nm is observed within the pH range of 2–4. At pH values below the optimal acidity, protonation of oNBAP occurs, leading to the dissociation of its complex with zirconium; at higher pH values, extraction efficiency decreases. For maximum incorporation of the metal into the mixed-ligand complex, the required concentrations of ligand and surfactant are 3.5×10⁻⁴ M and 4.11×10⁻⁴ M, respectively. To determine the most effective extractant for the mixed-ligand complex, the extraction capabilities of chloroform, dichloroethane, chlorobenzene, toluene, benzene, and hexane were investigated. The best extractant was found to be chloroform at a volume of 500 μL.
Although salt addition can facilitate the extraction of metal complexes into the organic phase in liquid-liquid extraction methods [6, p.13442], experiments were conducted using sample solutions with NaCl concentrations ranging from 0.0 to 3.0 mol·L⁻¹. The results showed that NaCl concentration had no significant effect on extraction efficiency. The influence of temperature and aqueous phase volume on extraction was also studied. It was observed that within the temperature range of 10–40°C and aqueous phase volume of 20–40 mL, and at a centrifugation speed of 3000 rpm, the organic phase separated more effectively.
The mechanism of the complex formation reaction was investigated. The introduction of the nucleophilic substituent –NO₂ group into the benzene ring leads to a bathochromic shift in the reagent’s absorption maximum. In acidic medium, the azoid form of the reagent is stable. When the amount of organic solvent is increased, the reagent’s maximum light absorption undergoes a hypsochromic shift, the quantity of the azoid form increases, and changes in absorption intensity are observed. However, without changing the amount of solvent, increasing the acidity of the medium can shift the equilibrium toward the formation of the quinonehydrazone form, and the absorption maximum can be shifted toward the long-wavelength region. During the complex formation reaction, the first hydrogen ion released from the reagent separates from the hydroxyl group located in the para position relative to the azo group. The hydrogen atom in the para-positioned hydroxyl group is highly mobile and easily dissociates from the reagent during complex formation. The ratio of components in the complex was studied using the Starik-Barbanel method, isomolar series, and equilibrium shift technique. In the mixed-ligand complex, the component ratio is Zr(IV):oNBAP:STAB=1:2:2. The probable composition of the complex can be represented as follows:
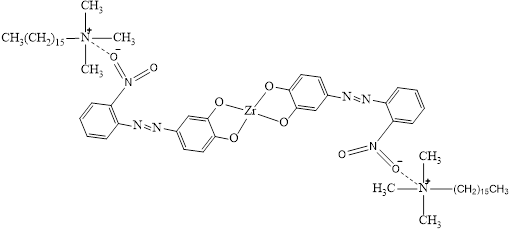
Numerous factors affect complex formation and extraction. To determine which factors have the greatest impact, extensive experimentation is necessary. To reduce the number of external experiments and assess the accuracy and reliability of the results, statistical calculations were performed. First, normality tests were conducted for the graphs showing the influence of factors on extraction yield. To identify which of the eight variables significantly affect the analysis results, the Plackett–Burman statistical method was applied. The statistically significant parameters were found to be ligand concentration (LC), pH, and volume of the extractant solution (VES). According to the statistical analysis, the optimal values of the independent variables for achieving maximum absorbance are pH = 3.3, LC = 3.5×10⁻⁴ M, and VES = 500 µL. The proposed method was successfully applied to determine microgram quantities of zirconium in natural and industrially significant materials.


