STUDY OF A GAS THERMOMETER
Журнал: Научный журнал «Студенческий форум» выпуск №36(303)
Рубрика: Технические науки

Научный журнал «Студенческий форум» выпуск №36(303)
STUDY OF A GAS THERMOMETER
Various phenomena associated with the heating and cooling of bodies occur in the world around us. They are called thermal phenomena. Thermal phenomena are physical processes that occur in bodies when they are heated or cooled. That is, these are the phenomena that occur with bodies as their temperature changes.
For example, when heated, cold water first becomes warm and then hot; a metal part removed from the flame gradually cools. We denote the thermal state of a body with the words "warm", "cold", "hot". A physical quantity, temperature, serves to quantitatively assess this state. The word "temperature" arose at a time when people believed that more heated bodies contained a greater amount of a special substance, caloric, than less heated ones. Therefore, temperature was perceived as the strength of the mixture of the body's substance and caloric. Before the invention of such a common measuring device in our everyday life as a thermometer, people could judge the thermal state only by their sensations: warm or cool, hot or cold. Temperature is a physical quantity that characterizes the thermal state of bodies [3].
Objective: to study the operating principle of a gas thermometer.
The relevance of this work is that studying a gas thermometer will help to more deeply study and understand the world around us, the properties of physical bodies and determine the value of temperature.
Tasks:
- get acquainted with the literature on this topic;
- assemble a model of a gas thermometer;
- test the model;
- formalize the measurement results.
A thermometer is a special device used to measure the temperature of a body, air, water and other objects or conditions. It is believed that this device was invented by the Italian scientist Galileo Galilei. The thermometer owes its modern appearance to the German physicist Daniel Gabriel Fahrenheit, and the Swedish astronomer and meteorologist Anders Celsius in the middle of the 18th century introduced two constant points: melting ice and boiling water. Finally, in the middle of the 19th century, the English physicist Lord Kelvin proposed to create an absolute temperature scale, where zero would not depend on the properties of the water or substance that fills the device. Currently, there are various types of thermometers: liquid, gas, mechanical, electronic, optical, infrared. Today we will consider a gas thermometer [4].
Due to the fact that gas is used as a filler, the measurement range increases. Such a thermometer can measure the maximum temperature in the range from -271°C to ~1000°C, depending on the gas used as a filler. This allows using gas thermometers to measure various hot substances [6].
At the end of the 18th century, 1787, Charles established that the same heating of any gas leads to the same increase in pressure, if the volume remains constant. When the temperature changes on the Celsius scale, the dependence of the gas pressure at a constant volume is expressed by a linear law. And from this it follows that the gas pressure (at V = const) can be taken as a quantitative measure of temperature. By connecting the vessel containing the gas to a pressure gauge and calibrating the device, you can measure the temperature according to the pressure gauge readings
Over a wide range of changes in gas concentrations and temperatures and low pressures, the temperature coefficient of pressure of different gases is approximately the same, so the method of measuring temperature using a gas thermometer turns out to be little dependent on the properties of a specific substance used in the thermometer as a working fluid. The most accurate results are obtained if hydrogen (hydrogen thermometer) or helium is used as the working fluid [2].
When measuring, the following points should be taken into account: deviations of the properties of the substance used from the ideal, changes in the volume of the cylinder with a change in temperature, the presence of impurities, diffusion, sorption (absorption of a substance from the environment) and desorption of the substance used by the surface of the cylinder, temperature distribution along the connecting tube [4].
The advantages of using such a thermometer include the following facts:
1. Wide measurement range. With this device, you can measure temperatures approaching absolute zero and readings from 2 to 1300 Kelvin (-270 to 1000 degrees Celsius). This meter is ideal for taking temperature readings of hot substances.
2. Accuracy. This type of thermometer is more accurate than a liquid one, its readings are considered the most accurate – accuracy from 0.003 to 0.02. Ideal performance is provided by the use of helium or hydrogen.
3. Weak dependence on the chemical nature of the gas. The temperature does not depend much on the chemical nature of the substance that is inside the device.
4. Easy to use. This type of device is easy to use.
5. Reliability. It is a very reliable device.
The disadvantages include the fact that such devices are non-compact and rarely used in everyday life. This is not a thermometer with a direct reading. It cannot accurately measure rapidly changing temperatures (measurement inertia) [4].
Gas thermometers are widely used in such scientific fields as physics and chemistry. In the temperature range where a conventional mercury thermometer can be used, the scale of a gas thermometer almost coincides with the scale of a mercury thermometer, since the temperature coefficient of gas pressure measured by a mercury thermometer, as we know, is almost constant.
Gas thermometers designed to measure low or not very high temperatures are made of glass or quartz and filled with hydrogen or helium. To measure temperatures below the liquefaction temperature of hydrogen, only helium can be used - the most difficult gas to liquefy. For very high temperatures (up to about 1500 °C), gas thermometers are made of a platinum-rhodium alloy that can withstand high temperatures, and filled with nitrogen (hydrogen is not suitable because it passes through heated platinum).
Gas thermometers are usually used only for checking and calibrating thermometers of other devices, which are more convenient in everyday use than gas ones. Calibration of ordinary thermometers is carried out in the bureau of standards, in metrology institutes and in individual physical laboratories. It is clear that when measuring temperatures with a gas thermometer, Charles's law must be followed absolutely precisely: after all, thermodynamic temperature is proportional to gas pressure by definition. Gas thermometers are often used in specialized laboratories. They are used to measure the work of other devices. This type of thermometer is used for relative measurement using a dual device.
The thermometer can be used to measure gas temperature when determining gas flow. This is also necessary if the measured and specified actual volume of gas needs to be converted to a standard volume. Actual volume is the volume at actual temperature and actual pressure. Standard volume of gas is the volume under normal conditions (T = 273.15 Kelvin = 0 °C, p = 1013.25 mbar).
Gas manometric thermometer TGP-100/160-TE/R is designed to measure the temperature of liquid and gaseous media.
Thermometers TGP-100/160-T/K-100/160-TE/K are used in the chemical, oil, gas, food, processing industries, as well as in water supply, heat supply, ventilation and air conditioning.

Figure 1. Gas-filled manometric thermometer TGP-100/160-TE/R
The gas thermometer indicating TGP is used to determine the heating level of water, oil and other liquids in °C, which do not have a negative effect on the thermal bulb, the pressure in which is not higher than 1.6 MPa. In addition, it monitors and regulates the electrical circuits of heating devices [1,5,8].
Objective of the work: to assemble and test a model of a gas thermometer.
Equipment: conical flask, beaker, thermometer, stopper with two holes, connector, transparent tube (2 pcs), holder, calorimeter, flexible tube, tripod.
Additional equipment and materials: aneroid barometer, vessel with warm and hot water, vessel with ice.
The operating principle of a gas thermometer is based on the dependence of gas pressure on it is temperature. According to Charles's law, the ratio of gas pressure to temperature in any state of the gas does not change if the volume and mass of the gas do not change during the transition from one state to another.
If we use the Celsius scale, this dependence can be expressed by the formula:
P=P0(1+αt),
P is the gas pressure at temperature t;
P0 is the gas pressure at temperature 0°C;
α is the temperature coefficient of pressure, which is the same for all gases and has a value of 1/273.15°C.
Therefore, the gas pressure can be used to determine its temperature.
A model of a gas thermometer can be made by using a flask sealed with a stopper with two holes, one of which is used to insert a thermometer, and the other - a connector. In this case, the air pressure in the flask can be measured with a water pressure gauge assembled, as shown in the figure, from two transparent tubes fixed vertically in a stand holder. Their lower ends are connected with a flexible tube. A ruler is attached between the tubes. The flask is connected with a second flexible tube to one of the elbows of the pressure gauge.
The volume of air in the flask can be considered constant. If its pressure is equal to atmospheric pressure, then the water levels in the two vertical elbows of the water pressure gauge are the same. When the pressure in the flask increases by ΔP, the water level in the right elbow will increase by Δh relative to the level in the left elbow. The values ΔP and Δh are related by the ratio:
ΔP=ρgΔh ,
where ρ is the density of water; g - acceleration due to gravity;
Δh - difference in water levels in the knees of the manometer.
The total air pressure in the flask is then equal to
P=P0+ΔP=P0+ρgΔh
(P0 is atmospheric pressure).
In order for the air volume to remain constant when the temperature changes, the left knee is raised or lowered until the water level in the right knee returns to its original position [7].
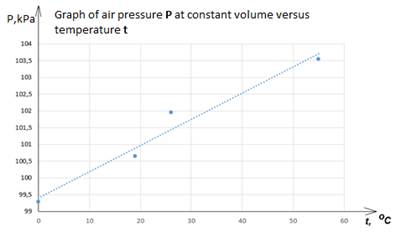
We calculate the pressure of three experiments using the formula and fill in the table:
P0 = P1 + ρgΔh.
t2 = 19°C (air temperature in the room)
t1=0 °C (ice temperature)
t3=26 C (warm water temperature)
t4=55 °C (hot water temperature)
P1 = 100641.5 Pa (atmospheric pressure as indicated by the aneroid barometer).
Table
Meanings
|
№ experience |
t,°C |
Δh0, m |
P, kPa |
|
1 |
0 |
0,155 |
99,292 |
|
2 |
19 |
- |
100,642 |
|
3 |
26 |
0,245 |
101,952 |
|
4 |
55 |
0,28 |
103,542 |
Measurement and calculation errors:
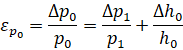
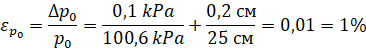
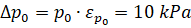
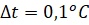
Based on experience and in accordance with Charles's law, the ratio of gas pressure to temperature in any state of the gas does not change. This principle is the basis for the design and operation of an industrial thermometer: a glass bulb connected to a tube. Liquid (mercury or colored alcohol) is poured into the bulb. Water cannot be used as a liquid for a thermometer due to its unusual expansion. A constant-volume gas thermometer is taken as a standard, and other thermometers are calibrated against it. The device is not compact and is rarely used in everyday life.
The goal has been achieved. A theoretical analysis of the available data on the operating principle of a gas thermometer has been carried out and the history of its creation has been studied. A model of a gas thermometer has been created and experiments have been conducted. The obtained dependencies fully confirm the theory, taking into account the error.

