Improving the technology of dairy drinks using the sugar beet pulp
Журнал: Научный журнал «Студенческий форум» выпуск №2(53)
Рубрика: Сельскохозяйственные науки

Научный журнал «Студенческий форум» выпуск №2(53)
Improving the technology of dairy drinks using the sugar beet pulp
Nowadays one of the actual directions of food industry is expanding the range of dairy products, improvement of production technology, development of new types containing various fillers enriched with vitamins, microelements [1].
The functional drinks are playing a essential role in disease prevention and wellbeing. These reduce rising burden on health care system through a continuous preventive mechanism. Drinks are no longer considered as thirst-quenchers; consumers are looking for specific functionality and it turns a part of their way of living. In last years these changes and developments have caused novel products. Sugar beet (Beta vulgaris subsp. vulgaris) is a part of the Chenopodiaceae family grown all over the world. Sugar beet contains a small amount of calories (just 45 kcal per 100 g) and has no cholesterol and little fat. Beet is a rich source of nitrates and several articles have shown its potential for significantly reducing blood pressure in humans. [2].
In modern conditions, one of the ways to intensify the food industry is the introduction of new low- and waste-free technologies and industries. This involves not only increasing the degree and completeness of processing of agricultural raw materials with more complete extraction of useful components from it, but also involvement in the national economic turnover of production waste in order to obtain additional commercial products from them.
To secondary raw materials can included beet pulp pallets, formed in a significant amount (70-90% by weight of beet) in the technological process of sugar production. The composition of pulp includes: up to 50% pectin, about 25-35% cellulose, hemicellulose- near 20%, 1,8-2,5% nitrogenous substances, about 1% of ash, 0,15-0,20% sugar, as well as vitamins (B1, B2, B6, C, etc.), enzymes, trace elements, small amounts phytosterols and fat. [3].
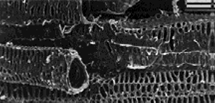
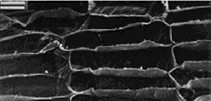
In the cell wall of sugar beet microfibrils are interconnected binding glycans whose length is 30–400 nm [4] (Fig. 1).
a b
Figure 1. Scanning electron micrograph of a cross section of sugar beet: a) an incision of one of the vascular bundles; b) phloem cut of the parenchyma. Scale 20 µm [5]
Pectin of sugar beet pulp pallets consist of pectose (protopectin), soluble pectin and polygalacturonic (pectin) acid. When hydrolyzed by enzymes or dilute acids, the insoluble part of pectins (protopectin) passes first into soluble pectin, and then into polygalacturonic acid. Available in the pulp esters are washed, thus cleaved methyl alcohol and acetic acid. Polygalacturonic (pectin) acid is devoid of oxymethyl and acetyl groups, so the salts of this acid are called pectates. Polygalacturonic acid consists of several molecules of galacturonic acid, interconnected by glucoside bonds. Carboxyl group of polygalacturonic acids are esterified by 60% methyl alcohol [6].
Nitrogen substances of pulp are mainly insoluble forms of protein (up to 80% of the total amount of nitrogen). Amide and ammonia nitrogen is completely converted into diffusion juice. Soluble nitrogen includes amino acid nitrogen, betaine, purine bases and nitrate. Proteins or proteins in the pulp are polymer molecules that consist of various amino acids.
In addition to simple proteins, the pulp contains a small part of complex protein-proteins, mainly nucleoproteins, that is, protein compounds with other high — molecular substances-nucleic acids. In the latter there are nitrogenous structural elements: purine, pyrimidine, as well as ribose (pentose) and phosphoric acid. Nucleoproteins are part of cell nuclei and are more difficult to digest than simple protoplasmic proteins. In raw pulp, the total content of amino acids is in the range of 0.3-0.5%. The amino acids include: alanine, valine, leucine, isoleucine aspartic and glutamic acids, lysine, arginine, phenylalanine, tyrosine, Proline and tryptophan.
Amides are contained in the roots of beet in a small amount. Amide nitrogen is found mainly in glutamine and asparagine. In addition to amino acids and amides, pulp contains betaine — "vegetable base", covering a number of nitrogenous compounds.
The fresh pulp contains about 19 mg/kg of vitamin C, dry pulp contains the following vitamins: B1 — 0,55, B2 — 0,20, B6 — 0,18, C — 5,0, Pantothenic acid — 0,21 and Biotin — 0,001. In pulp discovered two enzymes — pectinase and protopectins. It contains minerals, a small amount of fat, as well as two forms of sterols related to plant sterols or phytosterols. Up to 8.7% of palmitic acid, 36.1% of oleic acid and 18.6% of erucic acid associated with glycerine residues were isolated from crude pulp.
Table 1
The chemical composition of beet pulp of different types
|
Indicators |
Pulp, % |
|||
|
fresh |
pressed |
sour |
dried |
|
|
Dry matter |
6,0-9,0 |
14-20 |
11-15 |
86-93 |
|
Water |
91-94 |
80-86 |
85-89 |
7-14 |
|
Crude protein |
1,2-1,5 |
1,7-1,9 |
1,3-2,6 |
7-9 |
|
Crude Fiber |
3,5-4,5 |
5,0-7,0 |
2,8-4,2 |
19-23 |
|
Nitrogen-free extractives |
4,3-6,5 |
8,5-10,0 |
2,7-5,8 |
55-65 |
|
Ash |
0,6-1,0 |
1,1-1,4 |
0,7-1,8 |
2,4-4,3 |
|
Fat |
0,4-0,7 |
0,6-0,9 |
0,7-1,0 |
0,3-0,5 |
The most important biologically active substance of beet is betaine, the content of which is 1.9 - 2.4% depending on the variety. Betaine or trimethylaminuria acid (Fig. 1.) is an activator in the synthesis of phospholipids of cell membranes; has an effect on intermediate metabolism; reduces the level of homocysteine; normalizes the digestive system; activates lipid metabolism in the liver.
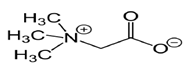
Figure 2. The structural formula of betaine
Also are interesting the beet pigments-betalaines, which have antioxidant properties. Betalains are water-soluble nitrogen-containing pigments, which comprise the red–violet betacyanins and the yellow betaxanthins.[7].
Betalains are contained in vacuoles of beetroot cells. Betacyanins are alkaloid plant pigments. The main structure of betacyanines consists of dihydroindole and dihydropyridine N-heterocyclic systems linked by sp2-hybridized carbon atoms. In most cases, natural beta cyanines are glycosides in which the C5 or C6 (but never both) of the dihydroindole ring is attached to a mono- or disaccharide to the OH group in the HOOC-NH-COOH + NHR22 position. Sugars may be acylated. Malonic, coffee, p-coumaric, ferulic, synapic and 3-hydroxy-3-methylglutar are the most common [8]. Betacyanins are absorbed in the range of 535 - 550 nm.
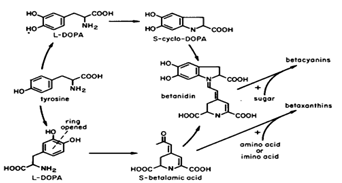
Figure 3. The main structure of betalain
Studies have shown that betalain pigments do not have a toxic effect on the human body. On the contrary, betacyanines have a beneficial effect on the body. These are:
- inhibition of the process of heme decomposition (the non-protein part of the hemoglobin molecule and cytochrome) [9];
- ensuring balanced redox processes involving fats [7];
- protection of erythrocytes from oxidative hemolysis [10];
-reducing the risk of diseases of the cardiovascular system [9];
-antiviral and antimicrobial effect [11];
- inhibition of the development of cancer tumors [12];
- participation in the process of activation of the enzyme quinone reductase;
- With chemotherapy, beta-cyanide has a powerful detoxicant effect [13].
Several reports have shown the potential of betacyanines as antioxidant pigments. Also studied their protective properties against oncology.
The aim of our research was to study the optimal extraction conditions to obtain the maximum amount of betacyanines. The use of betacyanines in industry is hampered by their instability. The stability of these pigments is highly dependent on factors such as pH, temperature, light intensity, the presence of metal ions, enzymes, oxygen. In Figures 4 and 5, one can see the dependence of the concentration of betacyanines on temperature and pH
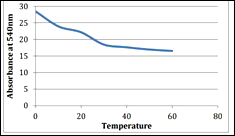

a b
Figure 4. Stability of betacyanines at different temperatures in the dark(a), in the light (b)
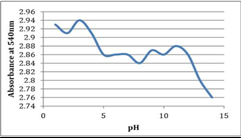
Figure 5. Stability of betacyanines at different pH
It should be noted that the content of beta cyanines in primary extracts depends on the extractant and is not the same in different solvents. Figure 6 presents the dependence of concentrations of betanin due to type of extractants and at figure 7 we can see stability of betacyanines in the two extracts, during 10 days storage.
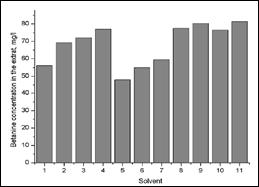
Figure 6. Betanine concentration in primary extracts
1 – dist.water; aq. solutions: 2 – citric acid sol. 1%, 3 – citric acid sol. 0.5%, 4 – citric acid sol. 0.2%, 5 – ascorbic acid sol. 0.1%, 6– ethanol sol. 50%, 7 – ethanol sol. 20%, 8 –sol. citric acid 0.5% and ascorbic acid 0.1%, 9 – sol. citric acid 0.2% and ascorbic acid 0.1%, 10 – sol. ethanol 20% and citric acid 1%, 11 – sol. Ethanol 20% and citric acid 0.5%
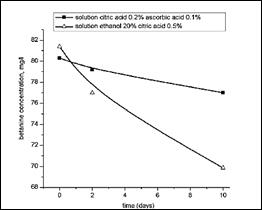
Figure 7.Variation of betanine concentration in the stored extracts at room temperature
In the figure 8 we can see the effect of ultrasonic action on the power of 30 %, 50% and 70% at the time of exposure to 1, 3, 5 minutes on the stability of betacyanins.
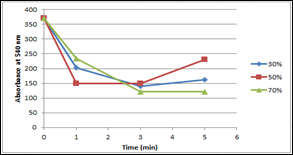
Figure 8. Stability of betacyanines at the effect of ultrasound
The analysis of the obtained experimental data allowed us to conclude that a solution of ascorbic and citric acid can be used for the stability of betacyanins in beet pulp extract. The process must be carried out at a temperature of 15-20C when exposed to sunlight and pH 3,9-4,2. Thus not using ultrasonic influence.
Conclusion. The obtained results show that the formed the waste of sugar production – sugar beet pulp – it can be used as an additive for antioxidant effects on the body.

